Note the BCCNM scope of practice for nurse practitioners was amended Jan. 3, 2022, to include prescribing of pharmaceutical alternatives for safer supply.
Prescribers and pharmacists are asked to add “SA” (safer alternative) to prescriptions and PharmaNet entries for prescribed harm reduction drugs. This will improve data for safer supply programs and identify unintended risks or harms.
Most prescribed alternatives to the toxic, illicit drug supply are also used for other indications (e.g., pain). Identifying prescriptions as SA allows programs run by the BC Centre on Substance Abuse, the Ministry of Mental Health and Addiction, and the Ministry of Health to monitor, evaluate, and better mitigate the opioid public health emergency.
Instructions for prescribers
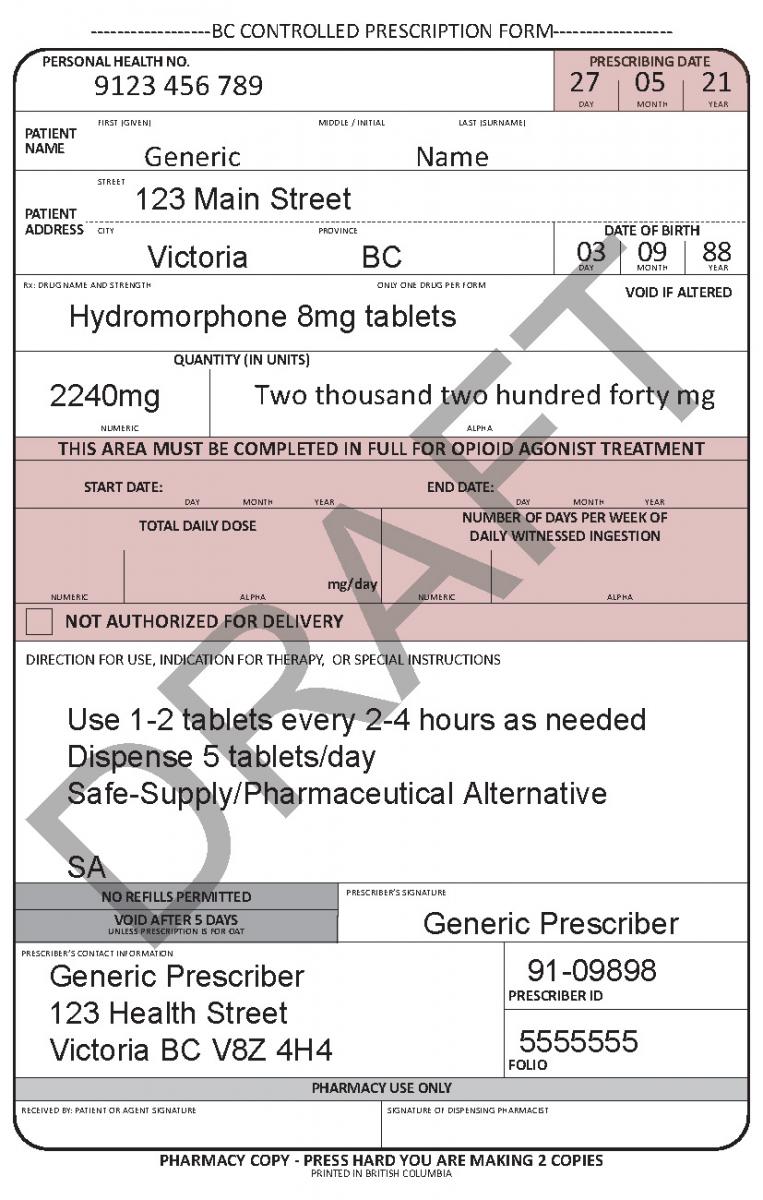
When writing a prescription for a drug to be used as an alternative to the toxic street supply (i.e., for risk mitigation during the dual public health emergencies or as a safer supply option), clearly add “SA” at the bottom of the Directions for Use section of the BC Controlled Prescription form. (See example)
“SA” tells the dispensing pharmacist to tag the prescription with a (non-public) identifying code, for program evaluation purposes, in PharmaNet.
Included drugs
The most common drugs prescribed for harm reduction are listed below, but all drugs prescribed for harm reduction should be identified with the “SA” code.
Opioids (not necessarily part of official OAT)
- Fentanyl patches, tablets, and inhalable compounded options
- Hydromorphone tablets, injectables, and inhalable compounded options, except when prescribed as part of a formal iOAT, or TiOAT treatment program
- Morphine injectable, and immediate or sustained release tablets/capsules, except when prescribed for OAT
- Oxycodone immediate and sustained release formulations
- Sufentanil injection
- Diacetylmorphine (DAM)*
*Currently DAM is not part of harm reduction programs. If any form (e.g., inhalable compounded options, injectable) becomes available for harm reduction, the prescriptions should include the SA code, which should be entered with each fill/part fill.
Stimulants
- Dextroamphetamine IR or SR
- Methylphenidate IR or SR
- Any other stimulants prescribed for harm reduction, either commercial or compounded
Benzodiazepines
- Diazepam
- Clonazepam
- Any other benzodiazepines prescribed for harm reduction, either commercial or compounded